Abstract
Should we be using hydrogen as a fuel? Does it matter if we produce hydrogen by reforming natural gas or electrolysis from renewable power?
References
In the spirit of transparency, here are the references.
- El-Shafie, M., Kambara, S. and Hayakawa, Y. (2019) Hydrogen Production Technologies Overview. Journal of Power and Energy Engineering, 7, 107-154. https://doi.org/10.4236/jpee.2019.71007
- electrolysis and reforming efficiency
- https://www.energy.gov/eere/fuelcells/comparison-fuel-cell-technologies
- hydrogen fuel cell efficiency
- Table 8.2. Average Tested Heat Rates by Prime Mover and Energy Source, 2007 – 2017, https://www.eia.gov/electricity/annual/
- This contains the heat rate for Combined Cycle and Simple Cycle Gas Turbine engines for power generation. We will need these numbers for comparing options.
- Cummins, https://www.cummins.com.ar/sites/bupa/files/pdf_g855gta855.pdf
- efficiency of a stock natural gas driven stationary engine
- I suspect this efficiency might be a little optimistic compared with a standard gas engine for transportation
Source code (Jupyter notebook) are available here.
Introduction
Hydrogen is proposed as a multipurpose fuel that will reduce our consumption of fossil fuels, and our production of CO2. It is proposed as a fuel for
- transportation
- blending with natural gas for combustion (https://balkangreenenergynews.com/turkey-to-blend-green-hydrogen-into-natural-gas-supply-network-for-heating/)
The chemistry for utilizing hydrogen is simple:
H2 + 2 O2 -> 2 H2O + ENERGY
And we have no CO2 emissions. But how is the hydrogen produced? Does the production method negate the zero carbon emissions for the end user? What options should we be comparing?
Basis
We will consider 100 GJ (lower heating value) of hydrogen as the basis. We will examine two ways to produce hydrogen:
- Steam methane reforming: CH4 + H2O -> pure H2 plus CO2 byproduct.
- Electrolysis of water with electrical power derived from a renewable source (wind, solar…)
We will consider two different uses for hydrogen.
- Motive power for a vehicle (using a fuel cell and a motor).
- Combustion heat for a home.
The following alternatives will be considered:
- Electricity production with combined cycle gas turbine (CCGT) and simple cycle gas turbine (SCGT).
- Standard gas engine running on compressed natural gas for powering a vehicle.
Data
Hydrogen generation efficiency.
| source | efficiency | fuel | references | |
|---|---|---|---|---|
| 0 | SMR | 0.75 | Natural Gas | El-Shafie, section 2 |
| 1 | Electrolysis | 0.70 | Electricity | El-Shafie, section 2.1 (56% – 73%) |
The efficiency values for CCGT and SCGT (from EIA) consider the higher heating value of natural gas. The Lower Heating Value for natural gas is about 10% lower than the higher heating value. This should not be enough to dramatically change the results.
| source | BTUkWhr | efficiency | |
|---|---|---|---|
| 0 | CCGT | 7649 | 0.44609 |
| 1 | SCGT | 11176 | 0.30531 |
The efficiency hydrogen powered transportation assumes 65% efficient alkaline fuel cell plus 90% efficient motor. The efficiency of a compressed natural gas engine is a stationary engine by Cummins. It is assumed that this efficiency value is reasonable for an engine in a road vehicle.
| Application | Hydrogen | Natural Gas | Notes | |
|---|---|---|---|---|
| 0 | Transport fuel | 0.585 | 0.295082 | 65% AFC + 90% motor |
| 1 | Combustion Heat | 1.000 | 1.000000 | both 100% |
H2 produced by SMR
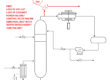
Steam Methane Reforming converts methane to hydrogen.
CH4 + 2 H2O + HEAT -> 4 H2 + CO2
A purification process is used to produce pure hydrogen and a CO2 byproduct. The CO2 byproduct could be recovered with standard processes. Note that carbon capture from SMR should be simpler than carbon capture from flue gas. This is because the CO2 is more concentrated in the reformer effluent (or hydrogen purifier waste stream) and there is no oxygen.
We will consider 100 GJ of H2 produced by steam methane reforming.
From the 75% efficiency of the SMR process, we need 133 GJ of natural gas to produce 100 GJ of hydrogen. There is a lot of waste heat available from this process, which could be useful for district heating or a business that requires heat. Who wants a SMR in their back yard?
We also need to consider the applications where we consume the hydrogen, both as useful shaft work in a vehicle and heat recovered for residential heating.
The amount of useful energy recovered from the hydrogen is:
| Application | Hydrogen | notes | |
|---|---|---|---|
| 0 | Transport fuel | 58.5 | shaft work |
| 1 | Combustion Heat | 100.0 | heat |
Now we consider replacing the hydrogen appliances with natural gas driven appliances. An efficient compressed natural gas engine would replace the fuel cell and electric motor. The amount of natural gas needed as a direct replacement would be:
| Application | NatGas Direct Use | NatGas for SMR H2 | |
|---|---|---|---|
| 0 | Transport fuel | 198.25 | 133.333333 |
| 1 | Combustion Heat | 100.00 | 133.333333 |
Our replacement engine would consume 198 GJ of natural gas. This is worse than consuming 133 GJ of natural gas to make hydrogen.
We might be able to make the arguement that reforming natural gas to produce vehicle hydrogen is a more effective use of natural gas, compared to simply using compressed natural gas as vehicle fuel.
Combustion heat would consume 100 GJ of natural gas in a furnace, which is better than consuming 133 GJ of natural gas to make hydrogen, and then burning in an equally efficient furnace to recover heat.
We should not burn the SMR hydrogen to provide heat.
Hydrogen from Electrolysis
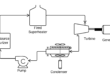
The other method to produce hydrogen is by electrolysis, and we will consider renewable electricity sources (wind, solar…) as our supply of electricity.
How much electricity is needed to produce 100 GJ of hydrogen? And what could we do with this new supply of electricity besides producing hydrogen? Again, we will consider that the hydrogen is ultimately used to power a vehicle or heat a home.
From the 70% efficiency of the electrolysis process, 100 GJ of hydrogen requires 143 GJ of electricity.
The simplest option for utilizing our additional renewable electricity supply is to reduce the load on a CCGT or SCGT power plant.
| source | energy | |
|---|---|---|
| 0 | CCGT | 320.242959 |
| 1 | SCGT | 467.908918 |
And we need to replace our consumers with simple natural gas burning appliances. We already have calculated this as the GJ of natural gas that is the direct replacement for consuming 100 GJ of hydrogen in these appliances.
| Application | NatGas Direct Use | NatGas for SMR H2 | |
|---|---|---|---|
| 0 | Transport fuel | 198.25 | 133.333333 |
| 1 | Combustion Heat | 100.00 | 133.333333 |
The net increase in fossil fuel consumption is the added demand from the appliances less the demand backed out of the fossil fired power generators. We have four different results depending on how we generate the power and how we use energy.
| Application | CCGT | SCGT | |
|---|---|---|---|
| 0 | Transport fuel | -121.992959 | -269.658918 |
| 1 | Combustion Heat | -220.242959 | -367.908918 |
We can use the additional power generated by the extra renewable sources to back out existing natural gas fired generating facilities. This decreases our net natural gas consumption, compared to using the electricity to generate hydrogen (for use as transport fuel or combustion heat). This also requires no additional infrastructure, such as the electrolysis units for generating hydrogen or the fuel cells for producing motive power.
Summary
The results were opposite to my “gut feel” about using hydrogen to decrease our consumption of natural gas. This is why we do engineering and let the evidence drive our decisions (and our emotions).
The hydrogen produced by Steam Methane Reforming might be a more effective transportation fuel than compressed natural gas. However, we need to evaluate this as an investment that reduces our energy consumption. The investment cost is that of the reformer (H2 production) and the fuel cells (transport power supply). This will decrease the energy cost for operating the vehicle, compared to standard technology of compressed natural gas for an engine. We need to be confident that the up-front cost has an acceptable Payback Period (say 5 years) compared to the current alternative. The calculations for Payback Period should consider cost in both the financial and energy terms.
Hydrogen producd by SMR is too valuable to burn as a fuel. Period.
Producing hydrogen with renewable electricity is a different story. We should not use renewable electricity to produce hydrogen. We should use our most efficient technology to back out our least efficient technology. When we consider renewable electricity in this manner, it makes more sense to back out existing gas turbine power plants compared to producing hydrogen as an erotic exotic fuel. When the very last gas turbine has been decommissioned, then it will be time to use renewable electricity to produce hydrogen. Hydrogen is a good end-game, but it is not a good strategy for the middle of the game.
Here, the question is not “Is hydrogen too valuable to burn” but “How should we use renewable sources of electricity?”