Introduction
Many of us use a natural gas fired furnace to maintains a comfortable 20 C in our homes. A modern natural gas furnace is typically 95% efficient or better.

If we go into an oil refinery we will find large furnaces (also gas fired) that are used to heat crude oil from 130 C to (say) 500 C.

The efficiency of modern refinery furnaces is usually over 80%, but rarely exceeds 85%. The efficiency of large furnaces in petrochemical plants and the boilers in thermal power generating stations are no better.
With over a hundred years of continuous improvement, why is an industrial furnace less efficient than the furnace in your home?
Let’s explore the truth.
Efficiency trends
How has the efficiency of industrial and residiental furnaces changed over the last 100 years? The efficiency of residential furnaces is fairly easy to find (Energy Solutions Center, 2008), and I have examples of low (60%) and mid (80%) efficient furnaces in my basement. I found the efficiency of a vintage 1920’s industrial coal fired boiler from an ASME document (ASME Landmark 185). The efficiency of industrial fired heaters since 1970 is from various projects that I have worked on.
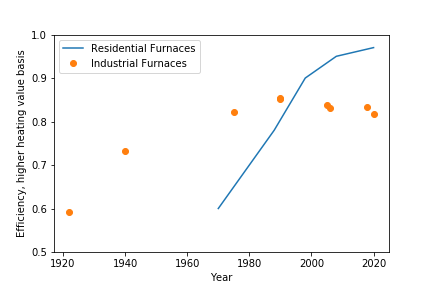
On the residential side, my old 1972 Lennox (60,000 BTU/hr) with B-vent should be around 60% efficient. Furnace efficiency rose throughout the 1970s and 80s, and by 2008 residential furnaces met or exceeded 95% efficient.
We need to go back a little further for industrial furnaces. A publication from ASME (ASME Landmark 185) shows that a coal fired boiler was about 60% efficient in 1920. This year is important because it marks the beginning of different strategies to improve efficiency. The method in this landmark case was to use a large, cumbersome device to recover the heat from hot flue gas and preheat combustion air. Simpler methods to improve efficiency were possible with improved fabrication methods for piping (the simple guts of all furnaces). Welded studs allowed piping to recover more heat, and this evolved into fins. From 1920 to the 1960s, there was steady innovation that increased furnace efficiency. My experience shows that industrial furnaces were over 80% efficient by mid 1970s, and I have a few references of 85% efficiency by 1990. There have been no increase in efficiency since.
History is great, but we we need to look at why refinery furnaces are less efficient than residential furnaces.
What goes on inside a residential furnace
A furnace has two sides: a hot side, and a cold side. We will start with the cold side.
This is my low efficiency, 1972 Lennox with the covers removed.

Cold air from our house is pulled into the furnace by the fan. For my old Lennox, the cold air is pulled into the bottom of the furnace. The cold air is pushed (upwards) past the heat exchanger (hidden): this is where the air warms up. The warm air leaves the furnace (top) at about 40 C.
The hot side is more interesting.

Natural gas is supplied by the 1″ black iron pipe on the left side at about 10 C. Combustion air for my old Lennox comes from the house at 20 C, and there is ducting to bring in fresh air (maybe at -20 C). For a high efficiency furnace, the combustion air goes directly from the outside into a combustion chamber.
Natural gas burns with the air and produces a flame with a temperature of about 2200 C under optimum conditions.

The flame looks nice, but they have not done anything useful for us. We have not recovered any heat. The flames flow into bottom inlet of the heat exchanger. The heat exchanger is nothing more than some large piping with hot combustion gas inside and cold air flowing around it. This is where we recover heat. Here is a top-down picture of the exchanger in the Lennox.

The hot gas from the flame flows into the heat exchanger, where it carries the hot gas across the large duct (possibly with two passes, hidden from view). The cooled gases leave through a vent (chimney).
The cold air flowing past the outside of the heat exchanger piping removes heat from the hot combustion gases that are flowing inside the heat exchanger piping. For my old Lennox, the combustion gases are still very warm (several hundred C) when they leave the heat exchanger. For a modern furnace, the flue gas is cooled from the flame temperature of 2200 C to 40 C (or lower), and the cool gas is blown outside. High efficiency furnaces condense some water and route this to a floor drain. You can see the floor drain at the bottom right corner of the first figure of this paper (3/4″ white plastic drain line).
The amount of heat produced by the furnace is related to the temperature of the flue gas. This figure shows the flame temperature for methane (where we recover no heat) and the amount of heat that is recovered when the gases are cooled as it flows through the heat exchanger (basis is burning 1 kg of methane).
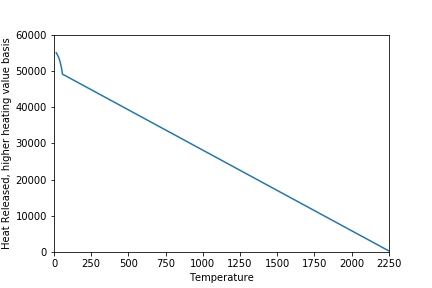
Efficiency is defined as the amount of heat recovered compared to the amount of heat that we could recover by cooling the flue gas down to 15 C and condensing all of the water vapour in the flue gas. Don’t ask my why we picked the arbitrary temperature of 15 C for the amount of energy that we could extract from a burning fuel (the heating value).
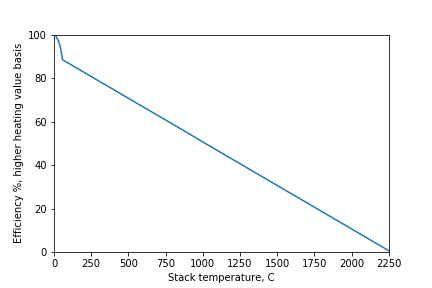
The flue gas temperature is reduced as we recover more heat. We will start condensing water at about 60 C. Note that there is a significant amount of heat available from condensing water if we cool the flue gas below 60 C. This was the incentive in the 1990s for recovering additional heat with residential furnaces.
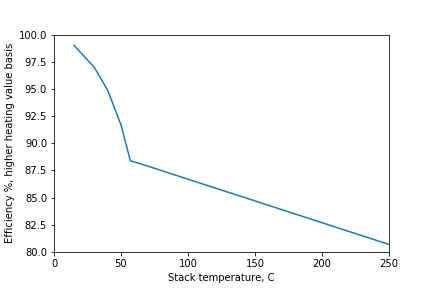
In the graph above, I calculate the efficiency to be 99% (not 100%) at 15 C. This discrepancy is due to a small amount of moisture that still exists in the flue gas at 15 C. The real world is not perfect.
Efficiency only depends on the stack temperature (to a pretty good approximation). Not how the furnace is designed or how large it is. Even the type of fuel (natural gas vs coal) plays a minor role in the efficiency. The next most significant effect is the amount of excess air for combustion. I am assuming that furnaces are operated using modern best practices for energy efficiency and minimizing the emission of unburned hydrocarbons or carbon monoxide (10% excess air).
We still have not said anything about the limits on efficiency.
The Second Law of Thermodynamics
The Second Law of Thermodynamics is a rather cumbersome way to state the obvious fact that heat flows from a high temperature to a low temperature. That’s why our house (at 20 C) gets cold in the winter. Our precious heat flows from our warm house to the cold outside. We beat this by burning a fuel (at a temperature higher than our 20 C house) and recovering heat.
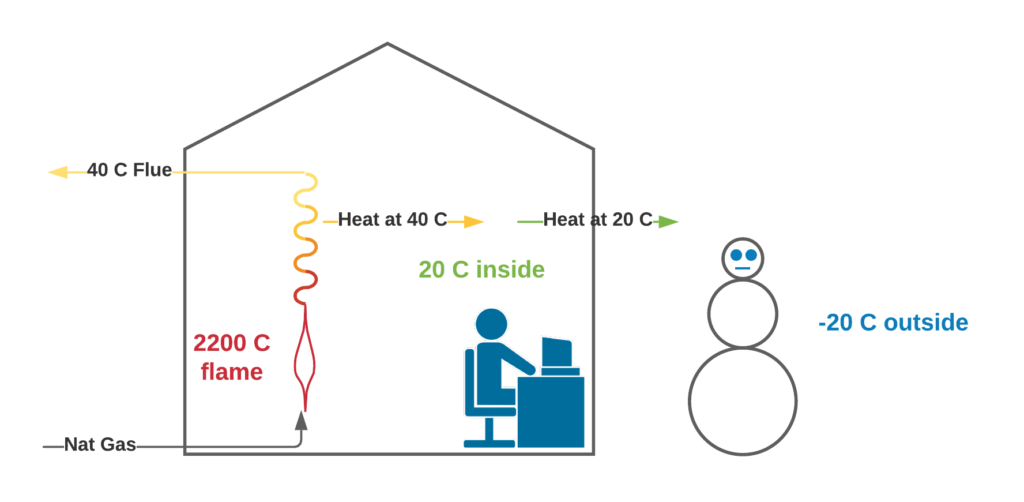
Lets take another look at our graph of furnace stack temperature and efficiency and see how this obvious fact is applied.
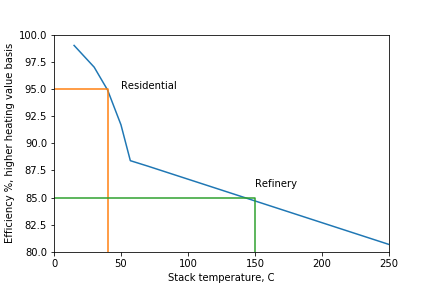
In our residential furnace, there is a huge flow rate of cold air at 20 C. In order to move heat to the cold air, the flue gas must be warmer than 20 C. A stack temperature of 40 C is pretty common in a modern furnace. Our furnace recovers 95% of the theoretical amount of heat because it is able to cool the flue gas to 40 C, and this is because the cold side is at 20 C.
But our refinery furnace is not used to heat up air at 20 C. It is used to heat up crude oil at 130 C. The flue gas must be, say, 20 C warmer than the feed temperature. That would be 150 C. By cooling of the flue gas to 150 C, our graph says that we will recover 85% of the theoretical amount of heat.
The efficiency of a furnace depends on the temperature of what we are heating up: the cold side temperature. Refinery furnaces are less efficient than residential furnaces because refinery processes are at a much higher temperature than simply keeping a house at 20 C. The refinery does not have a stream at 20 C where we can recover the remaining heat from the 150 C flue gas.
That is why a refinery furnace has a lower efficiency than a residential furnace.
Is there any way to improve this?
Stay tuned.
References
- Energy Solutions Center, 2008: https://naturalgasefficiency.org/for-residential-customers/heat-gas_furnace/
- ASME Landmark 185: https://www.asme.org/about-asme/engineering-history/landmarks/185-ljungstrom-air-preheater